Apria is contracted with most insurance companies and managed care organizations to provide home oxygen services, PAP, respiratory medications, and negative pressure wound therapy.
.
Medicare Product-Specific Requirements
Medicare Product-Specific Requirements
Home Oxygen Medicare Requirements
Home oxygen therapy is covered by Medicare only if all of the following conditions are met:
HOME OXYGEN DIAGNOSTIC REQUIREMENTS
Here is a list of some common health conditions that may qualify for coverage.
Please note that this list is meant to serve as a guide and is not all-inclusive of every covered condition.
- Severe primary lung disease such as:
- Chronic obstructive pulmonary disease
- Diffuse interstitial lung disease
Cystic fibrosis
- Bronchiectasis
- Pulmonary neoplasm, primary or metastatic
- Chronic bronchitis
- Emphysema
- Hypoxia-related symptoms/conditions that may improve with oxygen therapy, such as:
- Pulmonary hypertension
- Recurring congestive heart failure due to chronic cor pulmonale
- Erythrocytosis/erythrocythemia
LABORATORY EVIDENCE/QUALIFYING TESTING REQUIREMENTS
- Testing must be performed with the patient in a chronic stable state:
- As an outpatient: within 30 days prior to initial certification
- For patient transitioning from hospital stay to home: within two (2) days prior to discharge from an inpatient hospital stay to home
- For a patient in a skilled nursing facility or hospice: within 30 days prior to initial certification
- The qualifying blood gas study/oxygen saturation test was performed by a physician or qualified provider or supplier of laboratory services that is registered and able to bill for the test. Tests performed in a qualified provider’s office must be reviewed and signed by the patient’s physician or qualified provider.
- Medicare requires that test results be documented in the patient’s medical record and made available to the oxygen provider. If the test is not taken under these conditions, additional documentation must be obtained from the physician. These requirements apply to all Medicare oxygen patients, even if Medicare is in a secondary role.
NOTE
Patient’s chart notes must document the following:
- Documentation of the patient’s hypoxia-related condition and that his/her condition may improve with oxygen therapy.
- Documentation that other treatment measures have been tried and deemed ineffective (e.g., medications, inhalers, etc.).
PATIENTS CAN BE TESTED UNDER ANY OF THESE 3 REQUIREMENTS:
| Patient Tested on Room Air at Rest | Patient Tested During Exercise | Patient Tested During Sleep |
| A test taken during rest | A test taken on room air during exercise | A test taken during sleep |
|
Medicare considers oxygen medically necessary if SpO2 is less than or equal to 88% If SpO2 is greater than 88%, proceed to Step 2 |
If patient meets qualifying threshold of SpO2 less than or equal to 88% exercising, all three (3) of the required tests below must be performed within the same testing session and be recorded in the form of a medical record.
|
If a patient is tested during sleep, test must have at least two (2) hours of recorded time. Test must indicate arterial oxygen desaturation to 88% or less for at least five (5) minutes of testing period. A patient tested during sleep will not qualify for portable oxygen. |
NOTE
The oxygen saturation test results must include the name and address of the test facility (Independent Diagnostic Testing Facility (IDTF), hospital, physician office or sleep lab).If patient tested with exercise, 3 test results are required and all tests must be conducted during the same testing session:
- SpO2 _____% at rest on room air,
- SpO2 _____% with exercise on room air, AND
- SpO2 _____% on _____ LPM during exercise to show improvement ineffective (e.g., medications, inhalers, etc.)
Criteria for qualifying for oxygen include:
- Patient demonstrates SpO2 at or below 88% taken at rest, breathing room air, OR
- Patient demonstrates SpO2 at or above 89% taken at rest, breathing room air, AND
- Patient demonstrates SpO2 at or below 88% taken during exercise, breathing room air, AND
- it is documented that the patient’s SpO2 improves once the patient is placed on supplemental oxygen, OR
- Overnight Oximetry Test: Patient demonstrates SpO2 at or below 88 for at least 5 minutes, taken during sleep for a patient who demonstrated SpO2 at or above 89% while awake (nocturnal oxygen qualification only).
Regardless of test condition, the following values apply to all:
| Group I | Group II | Group III |
|
PaO2 ≤ 55 mm Hg or SpO2 ≤ 88% acceptable Recertification is required at 12 months from intial certification date |
PaO2 = 55-59 mm Hg or SpO2 = 89% acceptable only with secondary diagnosis of:
|
If PaO2 ≥ 60 mm Hg or SpO2 ≥ 90%, there is a presumption of non coverage |
If liter flow is greater than four (4) liters per minute (LPM), patient must meet Group I or Group II criteria while patient is receiving oxygen at a rate of 4 LPM or higher.
All patients must be tested in a chronic stable state, including those discharged from the hospital.
All coexisting diseases or conditions that can cause hypoxia must be treated and patient must be tested in a chronic stable state before oxygen therapy is qualified
OBSTRUCTIVE SLEEP APNEA (OSA) DIAGNOSIS REQUIREMENTS
If a patient with a chronic lung disease has also been diagnosed with obstructive sleep apnea (OSA), the test must be performed during the titration portion of a facility-based polysomnogram.
- Optimal treatment of OSA with the PAP device must be achieved.
- Titration must be conducted over a minimum of two (2) hours.
- During the titration phase, the patient continues to remain hypoxic (? 88% for a total of 5 minutes or more); and
- AHI/RDI reduced to ? 10 per hour; or
- If AHI/RDI was ? 10 per hour, titration demonstrates further reduction
PORTABLE OXYGEN REQUIREMENTS
- A portable oxygen system is covered if the patient is mobile within the home.
- Qualifying blood gas study must be performed at rest while awake or during exercise.
EMERGENCY ROOM TESTING
- A patient tested in an emergency room is generally not considered to be in a chronic stable state.
- If test was taken while patient was in the ER, patient must be tested again in physician’s office or by an Independent Diagnostic Testing Facility (IDTF).
SKILLED NURSING FACILITY, HOME HEALTH, OR HOSPICE TESTING
For initial oxygen qualification, patients may be tested while in a skilled nursing facility, or while under a home health or hospice stay if the test is taken while the patient is under a Medicare-covered Part A stay.
QUALIFIED TESTING PROVIDERS
- At rest: Hospital, skilled nursing facility, home health, hospice, on-site IDTF or physician’s office.
- During exercise: Hospital, skilled nursing facility, home health, hospice, on-site IDTF or physician’s office.
- During sleep: Hospital, on-site IDTF, physician’s office, Skilled Nursing Facility, Home Health Agency, or Hospice Organizations*
- Home sleep oximetry is limited solely to stand-alone overnight pulse oximetry performed in beneficiary’s home. Overnight oximetry performed as part of home sleep testing or as part of any other home testing cannot be used for oxygen qualification purposes.
- Apria cannot perform qualifying nocturnal oximetry studies, but can coordinate the tests with a qualifying IDTF.
*Testing performed by an SNF, Home Health, or Hospice requires verification of a “Part A Covered Stay” that coincides with the test date in order to be considered valid.
FACE-TO-FACE (IN-PERSON) EVALUATION FOR HOME OXYGEN
- Medical records must document the need and/or benefit of oxygen therapy for the patient, and must include the following:
A physician is required to document that the physician, physician assistant, nurse practitioner or clinical nurse specialist has conducted a face-to-face (in-person) examination prior to the written order prior to delivery (WOPD). - The documentation must be signed and dated by the treating physician or other qualified healthcare professional. That individual must have a National Provider Identifier (NPI) number and be enrolled in the Provider Enrollment, Chain and Ownership System (PECOS).
- For all oxygen equipment, the face-to-face evaluation must be conducted no more than 30 days prior to the initial Certificate of Medical Necessity (CMN) date.
Documentation that alternative treatment measures have been tried or considered and deemed clinically ineffective (i.e., medications, inhalers). - Documentation of the patient’s hypoxia-related symptoms/conditions.
- Documentation that the patient’s hypoxia-related symptoms/conditions may improve with oxygen therapy.
- Be legibly signed and dated.
NOTE
For recertification, documentation that an in-person visit took place within 90 days prior to CMN recertification is required.
WRITTEN ORDER PRIOR TO DELIVERY FOR HOME OXYGEN
At minimum, the written order prior to delivery must include:
- The patient’s name
- The physician’s name
- Date of the order and the start date, if start date is different from the date of the order
- The item of durable medical equipment or “DME” ordered, including:
- Route of administration (nasal cannula, via PAP or other)
- The specifics of varying oxygen flow rates (continuous, nocturnal or ambulating liters per minute)
- The length of need
- The prescribing practitioner’s National Provider Identifier (NPI)
- The signature of the ordering practitioner
- The signature date
- Helpful O2 Tips (RES-4131)
NOTE
Electronic signatures are acceptable if dated and there is an indicator to show that signature was electronically appended (by an approved CMS system).
Signature and date stamps are not acceptable.
CERTIFICATE OF MEDICAL NECESSITY
At Apria, we want to be sure that your patients get the equipment and services that they need. Please be sure to review this section carefully and refer to it frequently to make sure that your patient’s CMN is completed thoroughly and accurately. If you have any questions, don’t hesitate to call your local Apria Healthcare branch for guidance.
|
Cover Letter – Page 1 For your reference, the prescribing physician will be sent a cover letter with the information Apria was provided at the time of referral. The highlighted items are color coded to match the cover letter. |
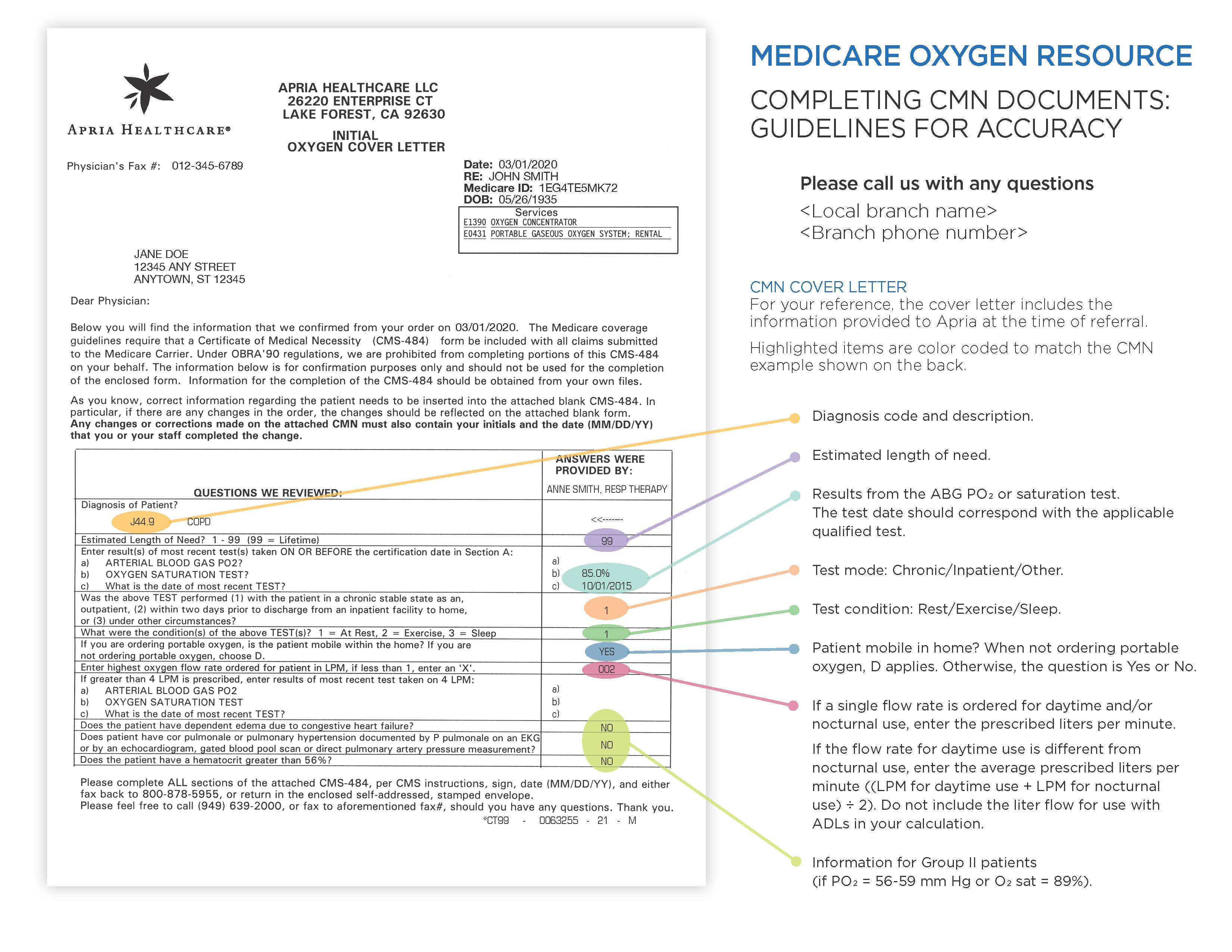
|
|
Cover Letter – Page 2 The highlighted items are color coded to match the Certificate of Medical Necessity (CMN). The cover letter can be used as a reference tool when completing the CMN. Please note that Section B of the CMN may not be completed by Apria. |
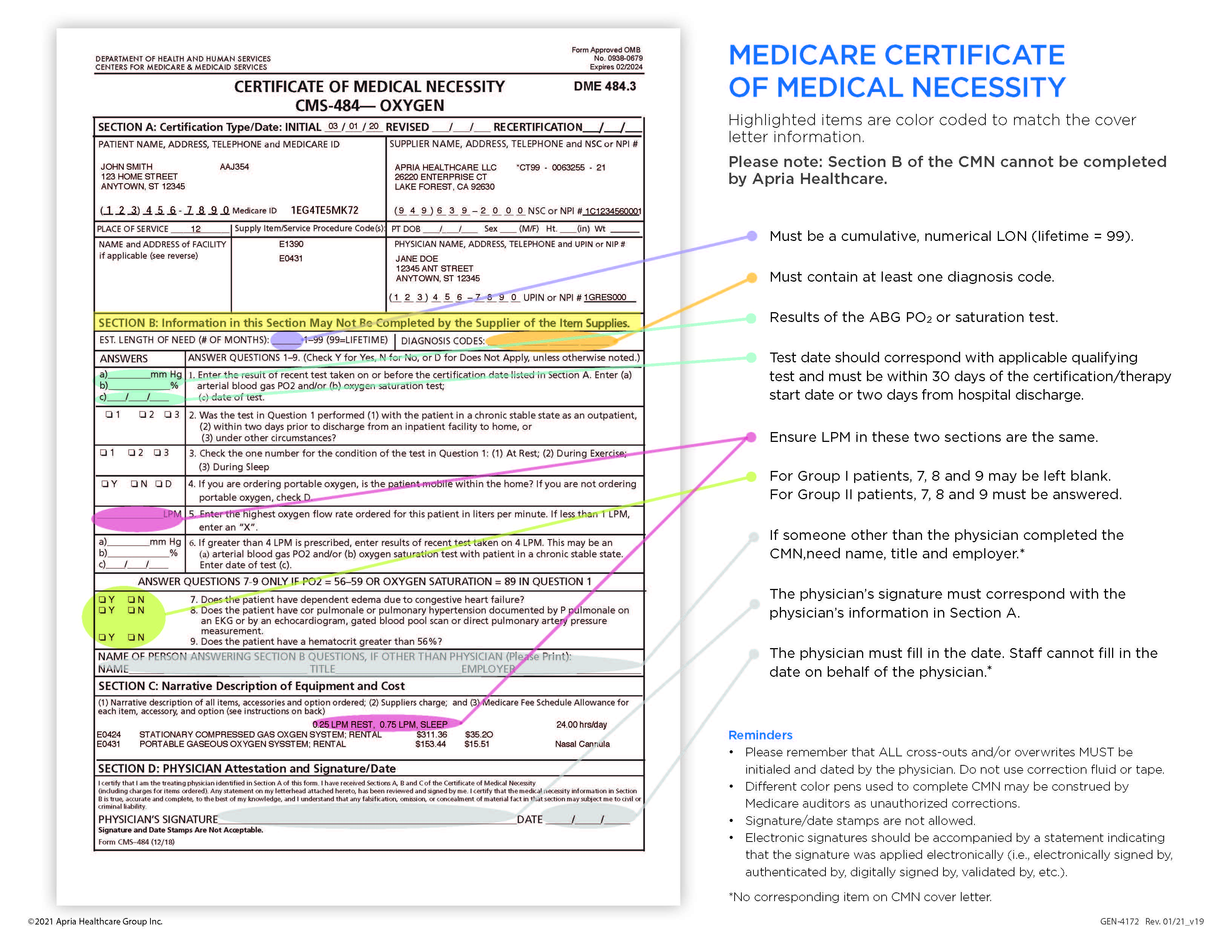
|
REMINDER
- All cross-outs and/or overwrites must be initialed and dated by the prescriber. Do not use correction fluid or tape
- Different-colored pens used to complete the CMN may be construed by Medicare auditors as unauthorized corrections
- Signature and date stamps are not allowed
- Electronic signatures should be accompanied by a statement indicating that the signature was applied electronically (e.g., electronically signed by, authenticated by, digitally signed by, validated by, etc.)
Downloadable Reference Tools
CMN Reference Tool (GEN-4172)
Medicare Part B — Oxygen Coverage (GEN-4173)
Medicare O2 Documentation Guide (GEN-4174)
Sleep Therapy Medicare Requirements
Please note that continuous positive airway pressure (CPAP) therapy is covered by Medicare only if all of the following conditions are met:
SLEEP THERAPY DIAGNOSTIC REQUIREMENTS
There must be documentation of a clinical evaluation prior to the sleep study that addresses signs and symptoms of sleep-disordered breathing.
A diagnosis of obstructive sleep apnea (OSA) for the coverage of a continuous positive airway pressure (CPAP) or bi-level device must be documented by:
- attended PSG performed in a sleep laboratory; or
- unattended HST home sleep monitoring device
LABORATORY EVIDENCE/QUALIFYING TESTING REQUIREMENTS FOR SLEEP THERAPY
The baseline sleep study conducted after the initial face-to-face evaluation must meet either of the following criteria for a positive diagnosis of obstructive sleep apnea:
- The Apnea-Hypopnea Index (AHI) or Respiratory Disturbance Index (RDI) is greater than or equal to 15 events per hour with a minimum of 30 events; OR
- The AHI or RDI is greater than or equal to 5 and less than or equal to 14 events per hour with a minimum of 10 events and documentation of:
- Excessive daytime sleepiness, impaired cognition, mood disorders, or insomnia; OR
- Hypertension, ischemic heart disease, or history of stroke
NOTE
The sleep study must be interpreted, signed and dated by a physician accredited in sleep medicine.
The sleep study, along with the interpretation of the results MUST be dated AFTER the initial face-to-face evaluation.
INITIAL COVERAGE OF NEW EQUIPMENT
1st Face-to-Face (In-Person) Evaluation
In addition to a positive diagnosis of obstructive sleep apnea via a facility-based or home sleep study, two (2) face-to-face evaluations are required for initial and continued Medicare coverage. The initial in-person examination must take place prior to the sleep study.
- A physician is required to document that the physician, physician assistant, nurse practitioner or clinical nurse specialist has had a face-to-face (in-person) examination with the patient in the six (6) months prior to the written order prior to delivery. Per the PAP Local Coverage Determination (LCD), for a new PAP patient, this visit must take place prior to the sleep study.
- The documentation must be signed and dated by the treating physician or other qualified healthcare professional. That individual must have a National Provider Identifier (NPI) number and be enrolled in PECOS.
The patient’s chart notes that reference the face-to-face (in-person) visit must document at least some of the following information:
- Sleep history and signs and symptoms of obstructive sleep apnea
- These include snoring, daytime sleepiness, observed apneas, choking or gasping during sleep, morning headache
- Duration of symptoms (how long the patient has experienced these symptoms)
- A standardized patient questionnaire which helps to assess the likelihood of sleep apnea
- Validated sleep hygiene inventory (this is a patient self-assessment tool, like the Epworth Sleepiness Scale or Berlin Questionnaire)
- Pertinent physical examination
- Focused cardiopulmonary and upper airways system exam and cardiopulmonary exam
- Body mass index
- Neck circumference, upper airway exam, and cardiopulmonary exam
NOTE
The initial face-to-face evaluation must be conducted PRIOR to the sleep test to assess the patient for obstructive sleep apnea (OSA).
INITIAL COVERAGE OF NEW EQUIPMENT
2nd Face-to-Face (In-Person) Evaluation
In addition to a positive diagnosis of obstructive sleep apnea via a facility-based or home sleep study, two (2) face-to-face evaluations are required for initial and continued Medicare coverage. The initial in-person examination must take place prior to the sleep study.
- Following the setup of the device, the patient must see the treating physician again, sometime between the 31st and 91st day, to document whether there has been improvement in the patient’s symptoms.
- The documentation must be signed and dated by the treating physician or other qualified healthcare professional. That individual must have a National Provider Identifier (NPI) number and be enrolled in PECOS.
- The patient’s chart notes that reference the face-to-face (in-person) visit must contain a review of the compliance data from the PAP device.
- For a patient to qualify for Medicare coverage, the device must document use at least 4 hours per night on 70% of the nights in a 30 consecutive day period during the initial 90 day trial period.
- Medicare coverage of the PAP device beyond the first 3 months is contingent upon demonstration of patient benefit from the use of a PAP device and the physician’s documentation of the follow-up face-to-face evaluation. Apria Healthcare will provide the physician with PAP usage information for Medicare beneficiaries.
WRITTEN ORDER PRIOR TO DELIVERY
The written order prior to delivery must include, at minimum:
- The patient’s name
- The physician’s name
- Date of the order and the start date, if start date is different from the date of the order
- The item of durable medical equipment or “DME” ordered, including
- Mask, pillows and other supplies needed (headgear, tubing, water chamber for humidifier, etc.)
- The specifics of the maximum and minimum pressure ranges and/or ramp time
- The length of need
- The prescribing practitioner’s National Provider Identifier (NPI)
- The signature of the ordering practitioner
- The signature date
NOTE
Electronic signatures are acceptable if dated and there is an indicator to show that signature was electronically appended (by an approved CMS system).
Signature and date stamps are not acceptable.
NOTE
If a PAP device is replaced during the 5-year reasonable useful lifetime (RUL because of loss, theft, or irreparable damage due to a specific incident, there is NO requirement for a new clinical evaluation, sleep test, or trial period.
If a PAP device is replaced after the 5-year RUL, there must be a new clinical evaluation by the treating physician that documents ongoing use of the PAP device.
A new order is always required for a replacement PAP device.
Negative Pressure Wound Therapy Medicare Requirements
CLINICAL DOCUMENTATION REQUIRED FOR WOUNDS
Additional clinical documentation is required in order for NPWT to be covered by Medicare and most insurance companies and will vary depending on the type of wound that is being treated. Below is a list of some of the documents that are required for each wound type.
Traumatic or Surgical Wounds
- Date of surgery or other incident that led to wound pre-operative report
- Post-operative report
- Additional supporting documentation indicating complications of surgically created wounds (e.g., dehiscence, flaps or grafts) and the medical necessity for accelerated formation of granulation tissue which cannot be achieved by other available topical wound treatments
Chronic Pressure Ulcer
- NPWT is only covered for Stage III or Stage IV pressure ulcers (Wound stage must be documented in patient’s medical record)
- Patient has been appropriately turned and positioned
- The patient’s moisture and incontinence have been appropriately managed (e.g., Foley catheter, bowel and bladder program)
- If the pressure ulcer is located on trunk or pelvis, documentation showing a low air loss or alternating air mattress (MUST BE group 2 or 3 support surface for Medicare) was tried or considered and ruled out prior to NPWT
- Duration of pressure ulcer (Include number of days in patient’s chart notes)
Diabetic/Neuropathic Ulcers
- Reduction in pressure on a foot ulcer has been accomplished with appropriate modalities
- The patient has been on a comprehensive diabetic management program (e.g., endocrinologist notes, diet, education provided, glucose readings, labs, etc.)
Venous Stasis Ulcers
- Documentation showing that compression bandages and/or garments have been consistently applied
- Documentation that leg elevation/ambulation was encouraged
NOTE
The patient’s chart must document the following:
- Quantitative measurements of wound characteristics, including wound length and width (surface area), and depth
- The amount of wound exudate (drainage)
- An assessment of wound healing progress
Medical records must include previous therapies tried or considered and why NPWT is essential for the patient
The patient’s chart notes must be updated at least on a MONTHLY basis and show a complete wound assessment including wound measurements.
IN-PERSON EVALUATION FOR NPWT
Medical records requested/obtained must document the need and/or benefit of NPWT for the patient, and must include the following:
- A physician is required to document that the physician, physician assistant, nurse practitioner or clinical nurse specialist has conducted a face-to-face (in-person) examination with the patient prior to the written order prior to delivery (WOPD).
- The documentation must be signed and dated by the treating physician or other qualified healthcare professional. That individual must have a National Provider Identifier (NPI) number and be enrolled in the Provider Enrollment, Chain and Ownership System (PECOS).
- For all NPWT equipment, the face-to-face evaluation must be performed for NPWT prior to the WOPD in addition to monthly wound assessments.
The patient’s medical records must include:
- Documentation of the wound history
- Previous treatment regimens (if applicable)
- Evaluation and care performed by a licensed medical professional
- A statement from the treating physician describing the initial condition of the wound, including:
- Complete wound measurements (length x width x depth and unit of measure)
- The date the wound measurements were taken
- The location of the wound
- Documentation of concurrent measures being addressed relevant to wound therapy, including:
- Application of dressing(s) to maintain a moist wound environment (including types of dressings and frequency of change)
- Debridement of necrotic tissue, if present
- Evaluation of provision for adequate nutritional status
- Length of sessions of use
- Dressing type and frequency of change
- Documentation of other modalities that have been tried and failed, or clear documentation stating why other modalities are being ruled out
- Examples of other modalities: debridement, proper support surfaces and/or removal of devices that are causing pressure, complete nutritional assessments with an increase of protein consumption, etc.
- Documentation to support specific clinical wound history
- Requirements differ for traumatic/surgical wounds, chronic pressure ulcers, diabetic/neuropathic ulcers or venous stasis ulcers
- Be legibly signed and dated
Home Enteral Nutrition Support Program Medicare Requirements
Medicare guidelines for home enteral nutrition
Medicare Part B: Covers services and supplies that are medically necessary to treat health conditions.
Enteral Nutrition
Enteral nutrition is covered under the prosthetic device benefit for Medicare Part B. The patient receives nutrition support through a tube placed into the stomach or small intestine. The tube may be nasoenteric, gastric, or jejunal.
Enteral nutrition is covered for a patient who has:
- Permanent non-function or disease of the structures that normally permit food to reach the small bowel or
- Disease of the small bowel which impairs digestion and absorption of an oral diet, either of which requires tube feedings to provide sufficient nutrients to maintain weight and strength commensurate with the patient’s overall health status.
Additionally:
- The patient must have a permanent impairment (ordinarily at least 90 days).
- Adequate nutrition must not be possible by dietary adjustment and/or oral supplements.
Disease of the small bowel which impairs digestion and absorption of an oral diet, either of which requires tube feedings to provide sufficient nutrients to maintain weight and strength commensurate with the patient’s overall health status.
- The patient must have a permanent impairment (ordinarily at least 90 days).
- Adequate nutrition must not be possible by dietary adjustment and/or oral supplements.
NOTE: Coverage may be possible for patients with partial impairments. For example: A patient with Crohn’s disease or a patient with dysphagia who can swallow small amounts of food.
Common Conditions
The following medical conditions are often associated with patients receiving enteral nutrition. This is a partial list that includes, but is not limited to, some common enteral-related diagnoses.
The patient’s condition can be either anatomic or due to a motility disorder. These medical conditions may meet coverage criteria if they cause impairment of consuming, digesting, and/or absorbing food. When the diagnosis itself does not reflect malabsorption or a functional impairment, additional documentation may be required to qualify a patient for enteral therapy coverage.
Medicare does not cover temporary impairment. The patient must have a permanent impairment (ordinarily at least 90 days).
Adult Hypertrophic Pyloric Stenosis
Allergic Gastroenteritis and Colitis
ALS (Amyotrophic Lateral Sclerosis)
Anoxic Brain Damage
Aspiration Pneumonia
Athetoid Cerebral Palsy
Atopic Dermatitis due to Food Allergy
Bloody Stools
Cancer
Carcinoma in Situ-Digestive Organs
Cerebral Palsy
Cerebrovascular Accident
Coma
Cow’s Milk Protein Allergy
Delayed Gastric Emptying
Developmental Disability
Diabetes Mellitus
Diarrhea
Dumping Syndrome
Dysphagia
Eosinophilic Esophagitis/Gastroenteritis
Failure to Thrive/Underweight
Gastritis
Gastroesophageal Reflux Disease
Growth Failure
Guillain-Barre Syndrome
HIV–AIDS
Hyperglycemia
Intestinal Malabsorption
Malabsorption
Malnutrition
Multiple Food Protein Allergy
Pancreatitis
Parkinson’s disease
Persistent Vegetative State
Pyothorax with Fistula
Reflux
Short Bowel Syndrome
Underweight
Additional Documentation Required for Enteral Feeding Pumps and Specialty Nutrients
Documented clinical rationale to justify the use of pumps or specialty nutrients is required in addition to documentation supporting medical necessity for enteral nutrition.
Pumps may be used as a result of complications associated with the use of the gravity or syringe methods of administration. Common conditions that may satisfy coverage criteria for enteral pumps:
- Reflux or aspiration
- Severe diarrhea
- Dumping Syndrome
- Administration rate < 100 mL/hr
- Blood glucose fluctuations
- Circulatory overload
- Use of Jejunostomy or Gastrostomy tube
Documented clinical rationale to justify caloric needs 750 or 2,000 is required in addition to documentation supporting medical necessity for enteral nutrition.
For patients who are prescribed calories outside of the 750 to 2,000 per-day range, documentation must support the clinical need. A Physician’s or Registered Dietitian’s assessment of estimated nutrition needs is ideal.
Use of formulas other than B4150 or B4152 require documentation of medical necessity to justify Medicare coverage. Justification for medical necessity is not diagnosis driven and may require supportive clinical laboratory information and/or clinical chart information for reimbursement.
Enteral Nutrient HCPCS (Healthcare Common Procedure Coding System) Descriptions
| HCPCS Code | Enteral Nutrient Categories |
| B4149 | Blenderized natural foods with intact nutrients |
| B4150 | Nutritionally complete with intact nutrients |
| B4152 | Nutritionally complete, calorically dense, (equal to or greater than 1.5 kcal/mL) with intact nutrients |
| B4153 | Nutritionally complete hydrolyzed proteins (amino acids and peptide chain) |
| B4154 | Nutritionally complete, special metabolic needs, excludes inherited disease of metabolism |
| B4155 | Nutritionally incomplete/modular nutrients |
| B4158 | For pediatrics, nutritionally complete with intact nutrients |
| B4159 | For pediatrics, nutritionally complete, soy based with intact nutrients |
| B4160 | For pediatrics, nutritionally complete, calorically dense (equal to or greater than 0.7 kcal/mL) with intact nutrients |
| B4161 | For pediatrics, nutritionally complete hydrolyzed/amino acids and peptide chain proteins |
Medicare documentation requirements for home enteral therapy
A written confirmation of a verbal order is required for home enteral therapy. Utilize Apria’s Fax Order Rx Form (ENT-4051), which includes the following:
- Beneficiary’s name
- Description or name of nutrients to be administered
- Method of administration (syringe, gravity, or pump)
- Equipment required to administer feed (pump/IV pole for pump feeds and IV pole for gravity feeds)
- Rate of administration
- If pump fed, rate needs to be documented in mLs/hr
- Length of need (# of months or 99 = lifetime)
- Treating practitioner’s signature with date, NPI, and printed name
- PECOS-certified ordering practitioner
- Start date of the order (if different than signature date)
- Current height and weight of patient
- Signature and date must be hand written or electronic (stamps are not acceptable)
Additional documentation requirements include:
- A copy of the patient’s medical record to support medical necessity for enteral nutrition in the home environment
- Additional documentation in the patient’s medical record is required:
- When the calorie need is <750 or >2,000 per day, or
- When an enteral pump is required, or
- When special nutrient formulas are required to meet unique nutrient needs for specific disease conditions
- Neither a face-to-face evaluation nor a written order prior to delivery (WOPD) is required prior to delivery; however, the patient must qualify for enteral based on the supporting medical records
Non-Invasive Ventilation
Non-Invasive Ventilator Coverage Criteria
Ventilators are covered for the following conditions:
Neuromuscular diseases, thoracic restrictive diseases, and chronic respiratory failure consequent to chronic obstructive pulmonary disease.
Medical Necessity for Non-Invasive Pressure Support Ventilation
Should include, but is not limited to documentation from the patient’s face-to-face evaluation and/or hospital medical records within the last 6 months that supports the disease progression leading to a need for non-invasive ventilation in the home environment.
This may include the following:
- Progress of the patient’s disease state
- Patient’s medical history and respiratory ailment
- Prior treatment results and current treatment plans
- If patient was previously on bi-level with or without rate as an outpatient, documentation of why the bi-level therapy is not sufficient for the patient
- For hospital discharge ONLY, the patient has completed a trial on the device that is being ordered
Apria Qualifying Testing Requirements Severe Neuromuscular Patients
Only if available:
- FVC test results, OR
- MIP/NIF test results
Severe Thoracic Restrictive Patients
Only if available:
- pCO2 test results, OR
- MIP/NIF test results
Chronic Respiratory Failure Consequent to COPD Patients
Requires one of the following:
- pCO2 ? 52 mm Hg or FEV1 ? 50% of predicted, OR
- pCO2 between 48–51 mm Hg or FEV1 ? 51–60% of predicted obtained with 2 or more respiratory related hospital admissions within the past 12 months
Face-to-Face1 (In-Person) Evaluation for Ventilation
Medical records must document the need and/or benefit of ventilation therapy for the patient, and must include the following:
- A physician is required to document that the physician, physician assistant, nurse practitioner or clinical nurse specialist has conducted a face-to-face (in-person) examination prior to the written order prior to delivery (WOPD).
- The documentation must be signed and dated by the treating physician or other qualified healthcare professional. That individual must have a National Provider Identifier (NPI) number and be enrolled in the Provider Enrollment, Chain and Ownership System (PECOS).
- For all ventilation equipment, the face-to-face evaluation must be conducted no more than 6 months prior to the written order
- Documentation that alternative treatment measures have been tried or considered and deemed clinically ineffective (i.e., medications, oxygen, respiratory assist device {RAD}).
- Be legibly signed and dated.
Written Order Prior to Delivery1 (WOPD) Requirements
In order for a WOPD to be valid, at a minimum, all elements listed below must be included on the WOPD. If one or more items are missing from the WOPD, the prescribing physician/practitioner must add the missing information. Any changes made to a WOPD must be initialed (or signed) and dated by the physician/practitioner and must be received prior to delivery.
The WOPD must include the following elements:
- Patient’s name
- Detailed description of the item ordered
- Prescribing physician’s name and National Provider Identifier (NPI)
- Signature and signature date of the prescribing physician (see additional clarification below)
- Date of the order
- For WOPDs created by the prescriber:
- A WOPD is acceptable if we receive the WOPD from the prescriber written on his or her desk top script pad and there is a date on the record along with a signature and all other critical elements, i.e., NPI, detailed description of the item, etc. The DME MACs will not be auditing for a separate physician’s signature date if the WOPD was created by the physician.
- Electronic WOPDs created by the physician are also acceptable providing there is an appropriate electronic signature identifier. For WOPDs created by the provider or by someone other than the prescriber
- If someone other than the physician (e.g., Apria employee, Registered Nurse (RN), hospital discharge planner) created any part of the order, the WOPD must include a separate order date and a signature and signature date from the physician. All approved Apria order forms contain spaces for the order date as well as a physician’s signature date.
Medicare Verbiage Discussing (Non-Invasive) Ventilation and RADs
“These ventilator-related disease groups overlap conditions described in the Respiratory Assist Devices LCD used to determine coverage for bi-level PAP devices. Each of these disease categories are conditions where the specific presentation of the disease can vary from patient to patient. For conditions such as these, the specific treatment plan for any individual patient will vary as well. Choice of an appropriate treatment plan, including the determination to use a ventilator vs. a bi-level PAP device, is made based upon the specifics of each individual beneficiary’s medical condition. In the event of a claim review, there must be sufficient detailed information in the medical record to justify the treatment selected.”2
1Detailed Written Orders and Face-to-Face Encounters – Released May 31, 2013, Medicare Learning Network
https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnmattersarticles/downloads/mm8304.pdf
2Correct Coding and Coverage of Ventilators – Revised May 2016, Noridian https://med.noridianmedicare.com/web/jddme/article-detail/-/view/2230715/correct-coding-and-coverage-of-ventilators-revised-may-2016 (December 03, 2015)


